The International System of Units (SI)
Officially known as the International System of Units (SI), the metric system is the international standard system of measurement units. It is based on the standard decimal number system, and is designed to be easy to learn, and simple to use.
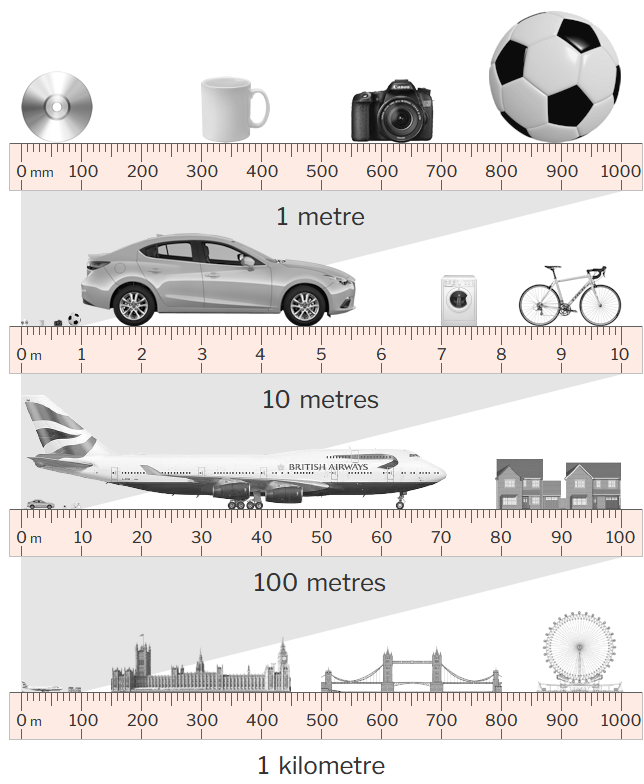
In everyday use, it is used to measure road distances and speeds, floor areas, storage volumes, energy consumption, and the mass and volumes of food and drink.
It is also used to measure temperature, electricity and the brightness of light bulbs. It is the standard system of measurement for international trade.
However, its application is not restricted to everyday use. Since its original inception, the metric system has evolved to become a single coherent system used for measurement in all fields of human endeavour, including science, medicine, technology, industry, commerce and sport.
Units
Units of measurement in the metric system relate to each other in a logical and coherent manner. Each quantity has one unit to measure it. This, together with the use of subunits based on multiples and submultiples of 10, make all calculations using metric units as straight forward as any other calculation using decimal numbers.

Base units
The metric system consists of a set of seven base units, for quantities including length, time and mass. All seven base units are defined from a set of seven constants which, in the SI, have fixed numerical values. Each defining constant is either an unvarying property of nature, or a technical constant.
Base units
| Name | Symbol | Quantity | Base unit |
| kilogram | kg | mass | 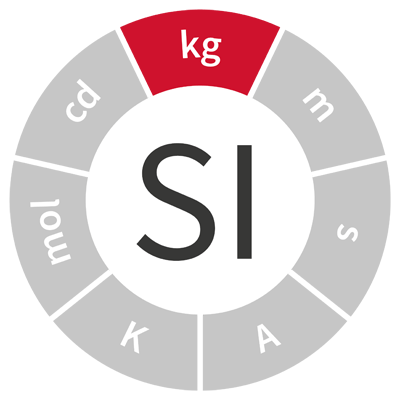 |
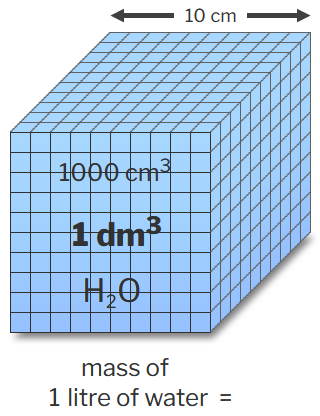
| The kilogram was originally defined as the mass of one cubic decimetre, or one litre, of water. | |||
| metre | m | length | 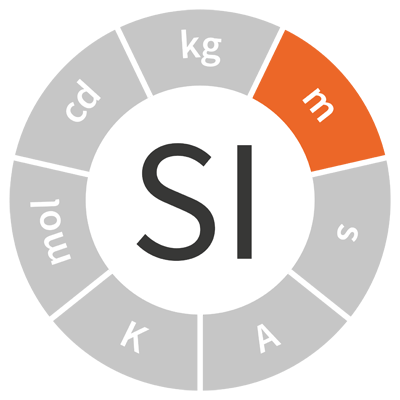 |
| The metre was originally defined as 1⁄10 000 000 of the distance from the North pole to the Equator. | |||
| second | s | time | 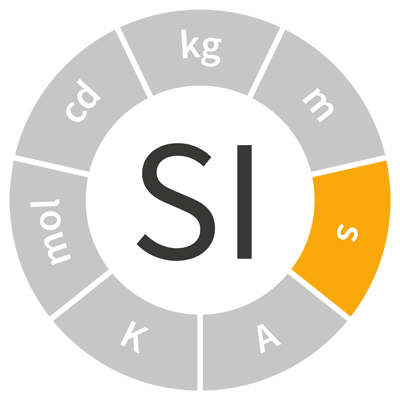 |
| The second was originally defined as 1⁄86 400 of a day. | |||
| ampere | A | electric current | 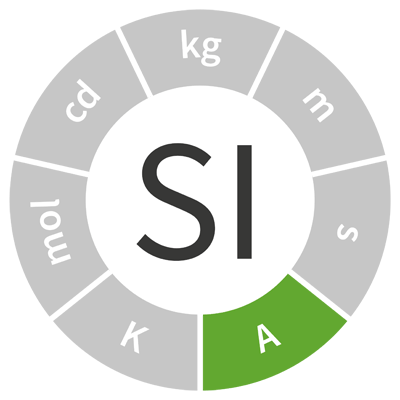 |
| kelvin | K | temperature | 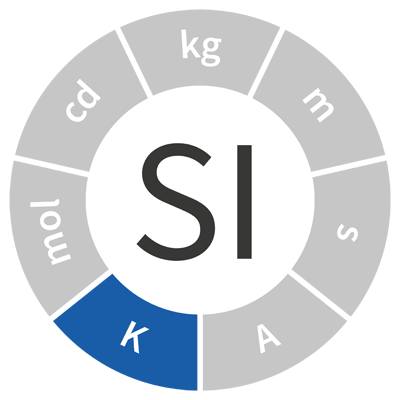 |
| A temperature difference of one kelvin is equal to one degree Celsius. The theoretically coldest temperature is zero kelvins, or -273.15°C | |||
| mole | mol | amount of substance | 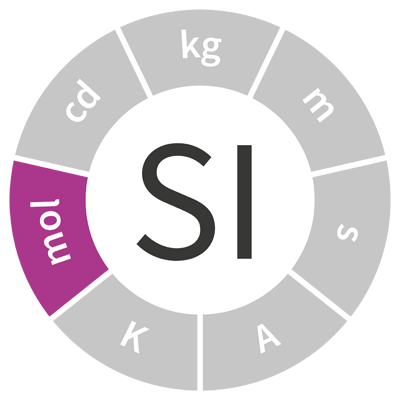 |
| The mole was originally defined as the number of atoms in 12 grams of carbon-12. | |||
| candela | cd | light intensity |  |
Derived unitsUnits for all other quantities are called derived units, and are defined as products of powers of one or more of the base units. Examples of derived units |
|||
| Name | Symbol | Quantity | Base units |
| square metre | m2 | area |  |
| The square metre is the SI coherent derived unit of area. | |||
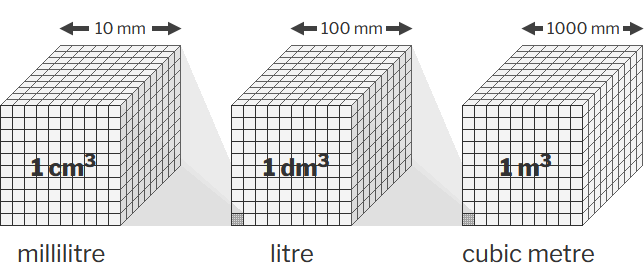
| cubic metre | m3 | volume |  |
| The cubic metre is the SI coherent derived unit of volume. | |||
| metre per second | m/s | speed |  |
| The metre per second is the SI coherent derived unit of speed. | |||
Derived units with special names and symbolsTo simplify their expression, some derived units have been given special names and symbols. Examples of derived units with special names and symbols |
|||
| Name | Symbol | Quantity | Base units |
| newton | N | force | kg m s-2 |
| The newton is the special name for the kilogram metre per second squared, symbol kg m s-2. |  |
||
| joule | J | energy | kg m2 s-2 |
| The joule is the special name for the kilogram metre squared per second squared, symbol kg m2 s-2. |  |
||
| watt | W | power | kg m2 s-3 |
| The watt is the special name for the kilogram metre squared per second cubed, symbol kg m2 s-3. |  |
||
| degree Celsius | °C | Celsius temperature | K |
| The degree Celsius is the special name for the kelvin, when using the Celsius temperature scale. |  |
||
Prefixes
The use of prefixes to denote decimal multiples and sub-multiples means that only one unit is required for any given quantity.
Common prefixes
| Prefix | Symbol | Value | ||||
| giga | G | 109 | 1 000 000 000 | |||
| mega | M | 106 | 1 000 000 | |||
| kilo | k | 103 | 1000 | |||
| hecto | h | 102 | 100 | |||
| deca | da | 101 | 10 | |||
| 100 | 1 | |||||
| deci | d | 10-1 | 0.1 | |||
| centi | c | 10-2 | 0.01 | |||
| milli | m | 10-3 | 0.001 | |||
| micro | µ | 10-6 | 0.000 001 | |||
| nano | n | 10-9 | 0.000 000 001 | |||
Subunits
Subunits can be constructed for any unit by combining any one of the standard set of prefixes with the unit name.
Examples of subunits
| Prefix | + | Unit | = | Subunit | Value |
| milli | gram | milligram | 0.001 grams | ||
| milli | metre | millimetre | 0.001 metres | ||
| kilo | metre | kilometre | 1000 metres | ||
| mega | watt | megawatt | 1 000 000 watts |
e.g. 10 000 metres can be expressed as 10 kilometres by combining the prefix ‘kilo’ (meaning 1000) with the unit name ‘metre’.
Symbols
In addition to names, each unit and prefix in the metric system has a unique symbol. Unlike abbreviations, symbols are independent of language and alphabet, and can be universally understood. Symbols are case sensitive.
Symbols for prefixes and units are combined in the same way as their names.
Examples of subunit symbols
| Prefix | + | Unit | = | Subunit | Value |
| m | g | mg | 0.001 g | ||
| m | m | mm | 0.001 m | ||
| k | m | km | 1000 m | ||
| M | W | MW | 1 000 000 W |
e.g. 10 000 m can be expressed as 10 km by combining the prefix symbol k (meaning 1000) with the unit symbol m.
Properties
The International System of Units (SI) is designed to be easy to use and widely applicable. It includes a number of desirable properties:
Units based on nature
Units in the SI are based on unchanging properties of the natural world. Originally the metre was based on the distance from the Earth’s North Pole to its equator, and the kilogram was based on the mass of water contained by a volume of 1⁄1000 of a cubic metre. Modern definitions of all SI units are more precise, but each unit is still defined in terms of one or more unchanging properties of nature, such as the speed of light in vacuum, or the charge on an electron.
Decimal system
The SI is a decimal measurement system. Calculations involving SI units are as straight forward as any other calculation using the standard decimal number system.
Standard symbols
All SI units have universally understood standard symbols. Any quantity expressed using SI units can be understood independently of language.
Rational system
The SI is a rational measurement system. Each physical quantity has only one SI unit. For example, length is measured using the metre, and time is measured using the second.
Prefixes and subunits
The SI defines a set of standard prefixes to denote decimal multiples and sub-multiples. Any SI prefix can be combined with any SI unit to form a subunit. The use of subunits provides flexibility and convenience when expressing quantities of different orders of magnitude in everyday use. For example; the use of the kilometre for road distances, and the millimetre for architectural plans.
Coherence
The SI is a coherent measurement system. A coherent system of units is a system of units based on a system of quantities in such a way that the equations between the numerical values expressed in the units of the system have exactly the same form, including numerical factors, as the corresponding equations between the quantities. A coherent derived unit is a derived unit that, for a given system of quantities and for a chosen set of base units, is a product of powers of base units with the proportionality factor being one.